Our Technology
Stemmatters has developed a versatile product development platform to support formulation development of medical devices and investigational medicinal products.
Stemmatters has established mimsys®, a technology platform consisting of an extensive library of proprietary, semi-synthetic new chemical entities derived from natural polysaccharide with tailorable physicochemical and biological performance.
mimsys® platform candidates demonstrate design features and characteristics which provide unique and highly competitive advantages over alternative biomaterial technologies available for regenerative medicine applications:
- Identity and purity: Complete structural identification and determination of chemical composition; Xeno-free.
- Manufacturing: Readily available starting materials and commodity reagents with defined specifications; Standardised manufacturing processes and consistent inter-batch reproducibility.
- Storage and stability: Off-the-shelf availability; Long-term stability under normal ambient conditions.
- Usage: Easy dissolution in sterile water at physiological temperature; Viscosity suitable for minimally invasive delivery or 3D printing applications; Proven compatibility with commercially available patient delivery systems.
- Performance: Tunable physicochemical properties; Biocompatible crosslinking mechanism; Mechanically stable and viscoelastic hydrogels providing volumetric filling; Controlled retention and/or delivery of active substances; Controlled degradation profile.
- Applications: Formulation of advanced therapy medicinal products; Formulation of implantable medical devices; Product applications requiring in situ delivery of active substances; Product applications in need of 3D environment supporting cell encapsulation and function.
Stemmatters adopts a rational approach to designing novel compounds that leverages advanced in silico modelling with a wealth of scientific and technical expertise.
Our experience with biomaterial development enables us to efficiently design, test and iterate on biomaterial candidates for specific use-cases and target product profiles. For each application, Stemmatters establishes a well-defined and structured development roadmap aimed at providing candidates for successful clinical development.
Stemmatters is strongly committed to expand product development collaborations with third parties. Our company is open to different collaboration frameworks. We encourage you to engage with us and begin the conversation.

The versatility and effectiveness of mimsys® is demonstrated by STM-148B, our lead candidate for cartilage repair. For developing STM-148B, design inputs considered user requirements and intended use, together with competitive differentiation against and intrinsic limitations of currently available products or other technologies under development.
STM-148B possesses important features that no other cartilage repair product in the market currently provides, offering significant competitive and usability advantages that benefit the patient as well as value based healthcare practices:
- STM-148B is animal-free and chemically defined. The molecule exhibits high manufacture reproducibility and ease of use. Upon formulation, STM-148B can be delivered during an arthroscopy, with minimum surgical invasiveness;
- STM-148B undergoes rapid crosslinking to form a stable and defect filling 3D hydrogel that possess excellent tissue adhesiveness, while dispensing use of any fixation aids;
- STM-148B hydrogels maintain viability and functionality of mammalian cells and further promote up-regulation of the expression of healthy chondrogenic extracellular matrix markers upon stimulation;
- STM-148B is highly biocompatible, which has been demonstrated by a comprehensive battery of in vitro and in vivo tests;
- STM-148B has excellent mid- and end-term local and systemic safety profile, demonstrated in ovine models of cartilage repair, either as a standalone medical device in combination with microfracture, or as a medical device for the delivery and retention of chondrogenic cells.

The mimsys platform is protected by four patent families:
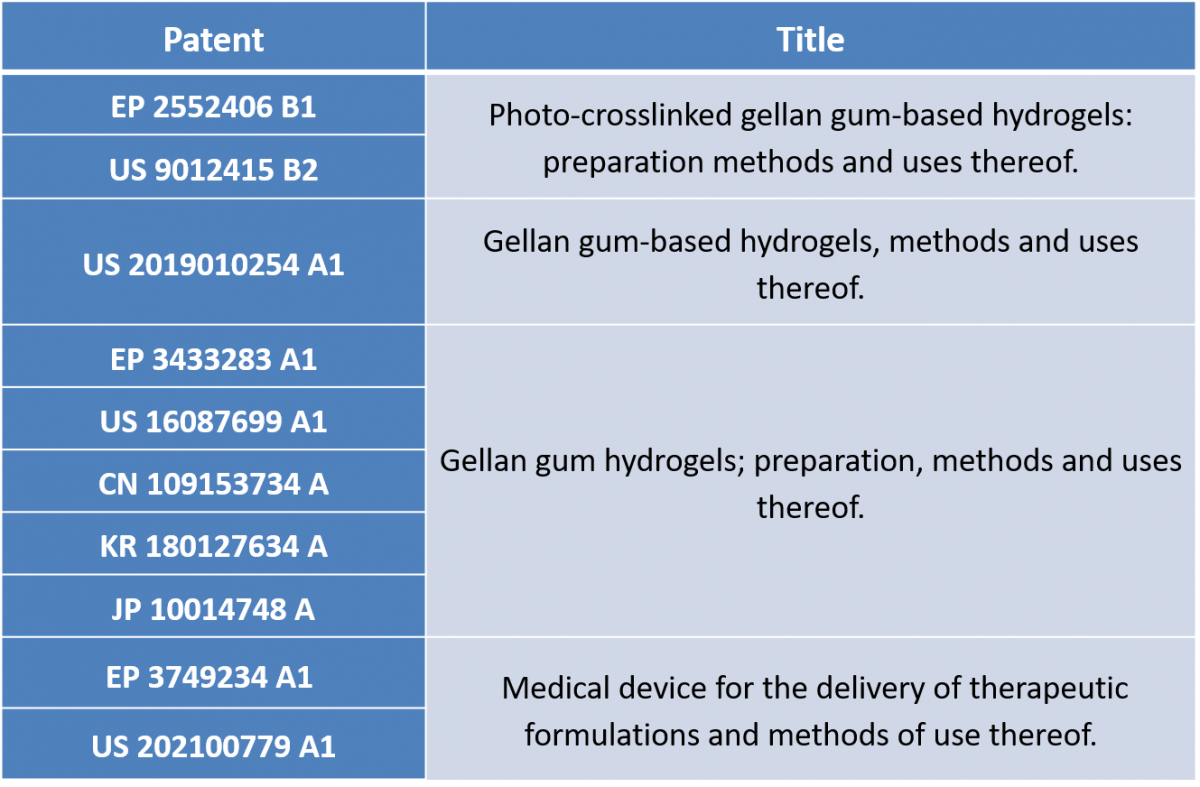
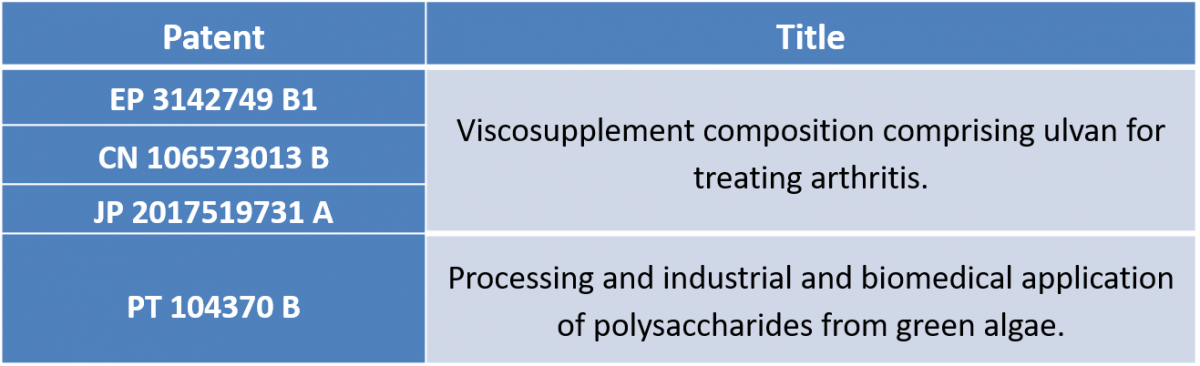
Stemmatters also owns strategic patents in areas adjacent to the mimsys® platform:
List of most relevant publications of our team:
-
P. Costa, D. Learmonth, D. Gomes, M. Cautela, A. Oliveira, R. Andrade, J. Espregueira-Mendes, T. Veloso, C. Cunha and R. Sousa. Mussel-Inspired Catechol Functionalisation as a Strategy to Enhance Biomaterial Adhesion: A Systematic Review. Polymer, 2021. doi: 10.3390/polym13193317.
-
D. Learmonth, P. Costa, T. Veloso, C. Cunha, M. Cautela, C. Correia, M. Vallejo and R. Sousa. Synthesis and Biological Evaluation of a Bioinspired, Tissue-Adhesive Gellan Gum-Based Hydrogel Designed for Minimally Invasive Delivery and Retention of Chondrogenic Cells. Biomater. Sci., 2020. doi: 10.1039/D0BM00286K.
-
C. Cunha, R. Andrade, T. Veloso, D. Learmonth, J. Espregueira‑Mendes and R. Sousa. Enhanced microfracture using acellular scaffolds improves results after treatment of symptomatic focal grade III/IV knee cartilage lesions but current clinical evidence does not allow unequivocal recommendation. Knee Surg. Sports Traumatol. Arthrosc., 2020. doi: 10.1007/s00167-019-05832-5.
-
L. Rocha, E. Gomes, J. Afonso, S. Granja, F. Baltazar, N. Silva, M. Shoichet, R. Sousa, D. Learmonth and A. Salgado. In vitro evaluation of ASCs and HUVECs co-cultures in 3D biodegradable hydrogels on neurite outgrowth and vascular organization. Front. Cell Dev. Biol., 2020. doi: 10.3389/fcell.2020.00489.
-
G. Soares, D. Learmonth, M. Vallejo, S. Davila, P. Gonzalez, R. Sousa and A. Oliveira. Supercritical CO2 technology: The next standard sterilization technique? Materials Science & Engineering, 2019, C99, 520–540. doi: 10.1016/j.msec.2019.01.121
-
C. Vilela, C. Correia, A. Morais, T. Santos, A. Gertrudes, E. Moreira, A. Frias, D. Learmonth, P. Oliveira, J. Oliveira, R. Sousa, J. Espregueira-Mendes and R. Reis. In vitro and in vivo performance of methacrylated gellan gum hydrogel formulations for cartilage repair. J. Biomed. Mater. Res. A., 2018, 106(7), 1987-1996. doi: 10.1002/jbm.a.36406.
-
C. Vilela, C. Correia, J. Oliveira, R. Sousa, J. Espregueira-Mendes and R. Reis. Cartilage Repair Using Hydrogels: A Critical Review of in vivo Experimental Designs. ACS Biomater. Sci. Eng., 2015, 1(9), 726-739. doi: 10.1021/acsbiomaterials.5b00245.
Download our company service catalog here.
Our company service catalog provides useful further information about our company and service offering. You are welcome to share the document with your colleagues as you please.
Please click this link to download the catalog .
We would also be very happy to post you a hard copy free of charge upon request .




